Case Study

Parker supports the design, manufacturing, and assembly of an innovative cancer therapy treatment device, utilizing advanced life science materials and technologies.
Application
One of three most common cancers for women is breast cancer, and every year hundreds of thousands of people diagnosed with cancer come face-to-face with the reality of radiation therapy – a process that can be taxing on the body, ultimately spawning other side-effects, from anemia and hair loss, to nausea and constant fatigue.
Parker has enjoyed a long-term relationship with an innovative San Jose, California company that develops cancer therapy devices and equipment. In cases where breast cancer is detected early, the device is used in a radiation therapy treatment option for patients undergoing lumpectomy procedures. But what's unique about this treatment option is the device delivers intraoperative radiation therapy (IORT) –where radiation therapy takes place within the same operative procedure. Utilizing the the same surgical incision used for the lumpectomy, the oncology surgeon inserts the radiation therapy applicator. The device's balloon catheters provide the channel for the miniaturized x-ray source to deliver isotope-free, high dose rate, low energy radiation treatment.
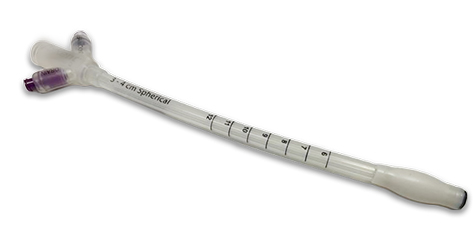
FEATURED PRODUCT: Multi-component cancer therapy treatment medical device
Challenge
The customer's applicator medical device is comprised of multiple components – individually designed for flawless function – which also need to be expertly engineered for optimal manufacturability, trouble-free overmolding, assembly, and secondary operations including screen-printing, EO sterilization, and packaging.
Application: Interoperative radiation therapy (IORT)
Parker Solution
“Oftentimes a potential partner comes to us with an idea, without understanding silicone product manufacturing,” said Sean Cannon, Medical Systems Business Value Stream Manager. “We offer partners a unique product mix wherein we are actually building something good together,” Sean added.
We worked with our customer, every step of the way to transform their idea into a finished device. In addition to our expertise in manufacturing components and tubing from a range of top rated medical grade silicone materials, our range of technology solutions for the customer included:
- Extruded tubing featuring
- Customized multi lumen configuration
- Parylene coating
- Liquid silicone molding (LSR) of
- Balloon
- Hub overmolding
- Drain plug
- Inflation port
- Assembly utilizing RTV adhesive
- Full functional leak testing
- Screen printed part markings
- Packaging
- Availability of EO sterilization

Parker's wide range of life science materials and technology solutions for finished medical devices
Parker CSS Division is currently working with the customer on various extended tube applicator specifications and sizes, as part of their product line.
Contact
Parker Hannifin
Composite Sealing Systems Division
7664 Panasonic Way
San Diego, CA 92154
(619) 661-7000
www.parker.com/css
NEED ENGINEERING ASSISTANCE?
