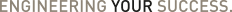Problem Solved!
Application Success Stories
From initial concept to production, Parker's engineering teams support many of the world’s leading manufacturers in the ever changing trends of the industry, helping them to expand their geographical footprint and achieve optimal operational efficiency.
Enhancing Wearable Drug Delivery Device Performance with Internally Lubricated Sealing Solutions
Parker is revolutionizing the performance of wearable drug delivery devices with their cutting-edge internally lubricated compounds. Overcoming significant design and friction challenges, these innovative seals ensure consistent, low-friction operation without external lubrication, adhering to stringent Life Science standards. By transitioning from the harder E1244-70 to the more installation-friendly EJ243-45, manufacturers now enjoy reliable drug delivery over the device's lifespan, meeting both FDA and USP Class VI approvals. Explore the seamless integration of advanced sealing technology into life-critical applications.
Sealing Challenge:
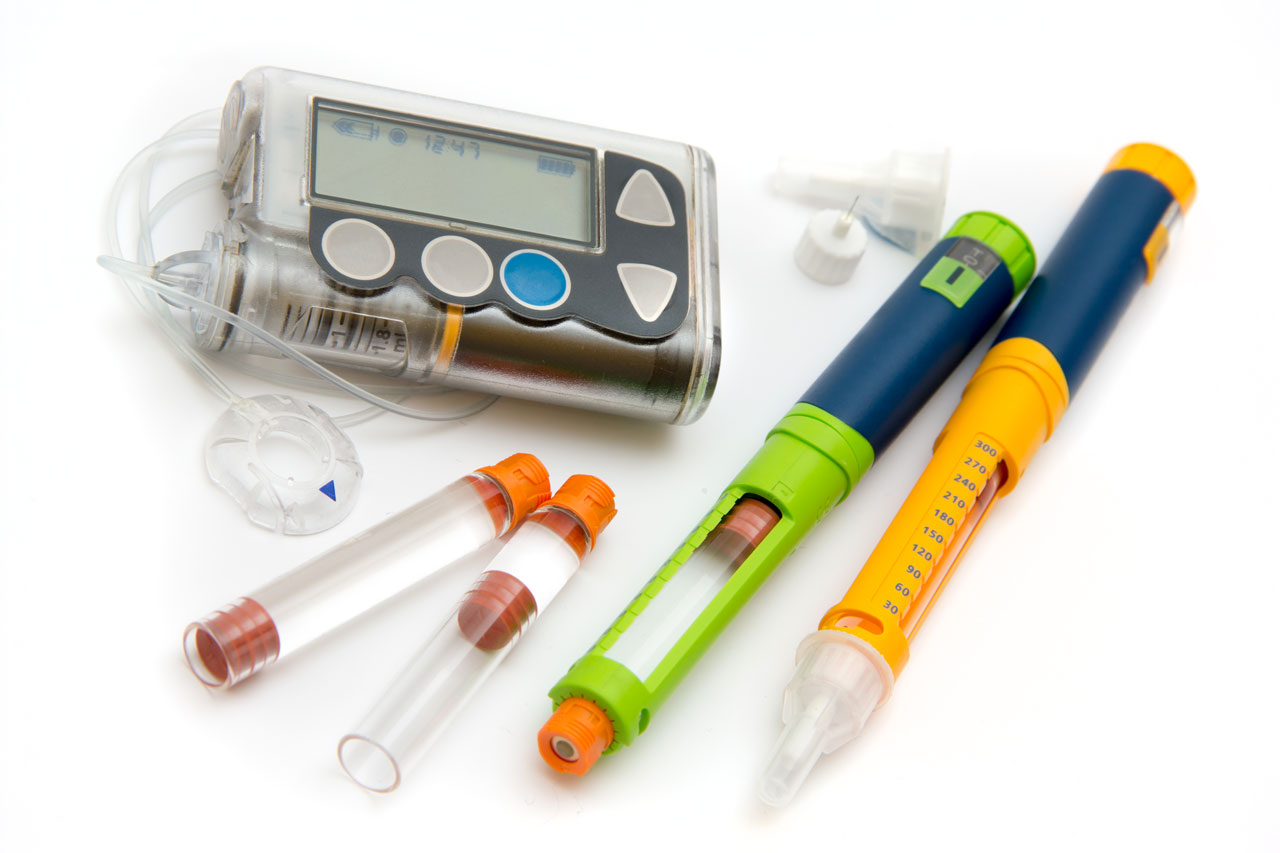
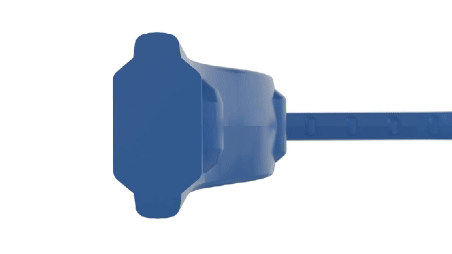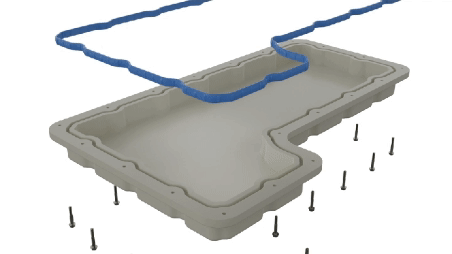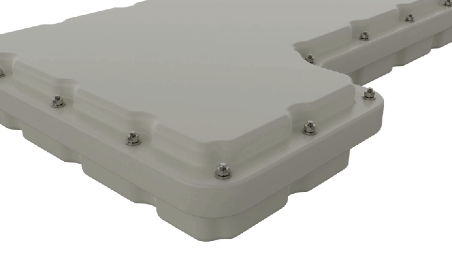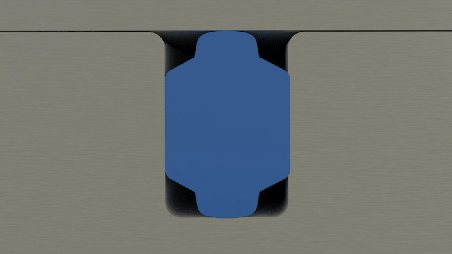
A manufacturer specializing in wearable drug delivery devices identified a critical issue affecting the device's performance - inconsistent drug delivery throughout the device's lifecycle. This inconsistency was traced back to the device's sealing system, which was unable to maintain optimal friction levels due to design constraints. Specifically, the device's groove and hardware configuration could not be altered to achieve a lower friction profile. Additionally, the application's strict adherence to Life Science standards precluded the use of external lubricants, complicating the search for a viable solution.
The challenges identified were:
- Design Limitations: The inability to modify the existing groove/hardware design to reduce friction.
- Friction Management: The need to achieve and maintain low friction in dynamic sealing applications without external lubrication.
- Life Science Compliance: The requirement for any solution to meet stringent Life Science standards, including ISO 10993 and USP Class VI approvals, to avoid contamination or adverse reactions.
Sealing Solution:
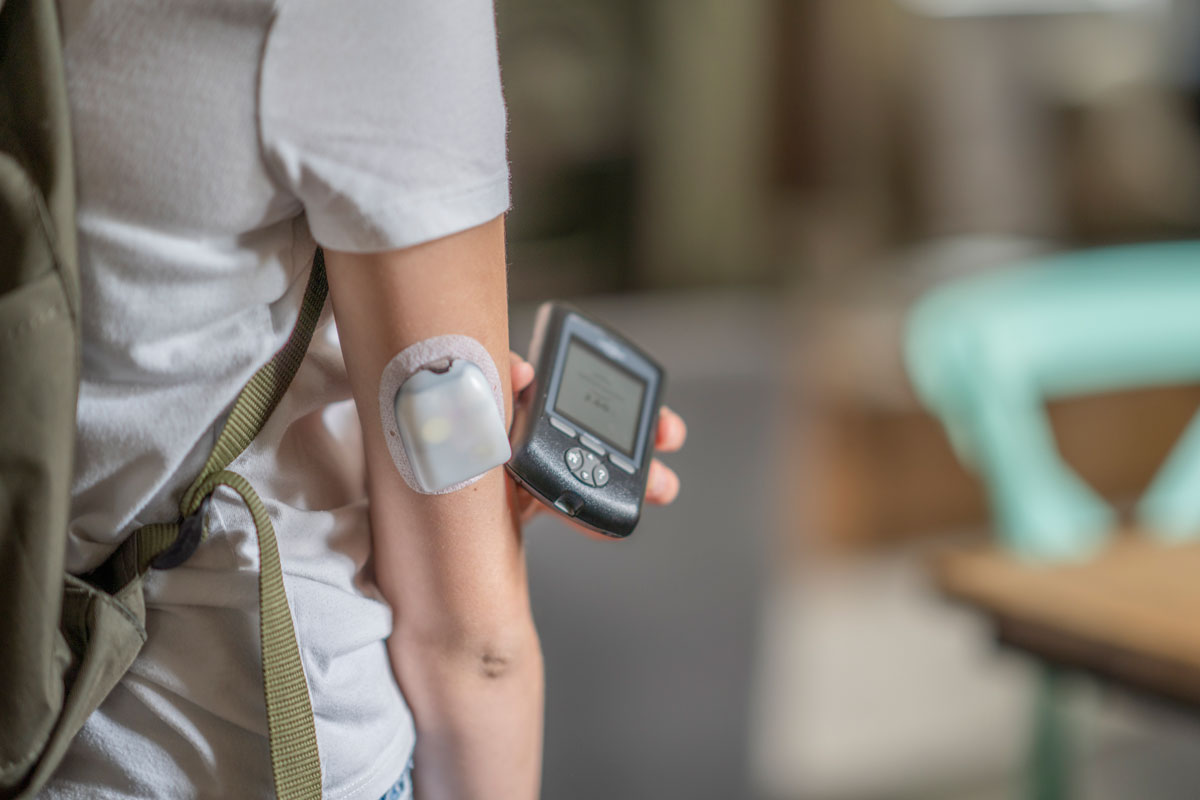
Parker O-Ring & Engineered Seals (OES) Division addressed these challenges by introducing a line of internally lubricated compounds designed specifically for dynamic sealing applications requiring consistent low friction over extended periods. These compounds release a wax-like substance over time, ensuring the seal remains lubricated throughout its lifespan, thereby facilitating consistent drug delivery.
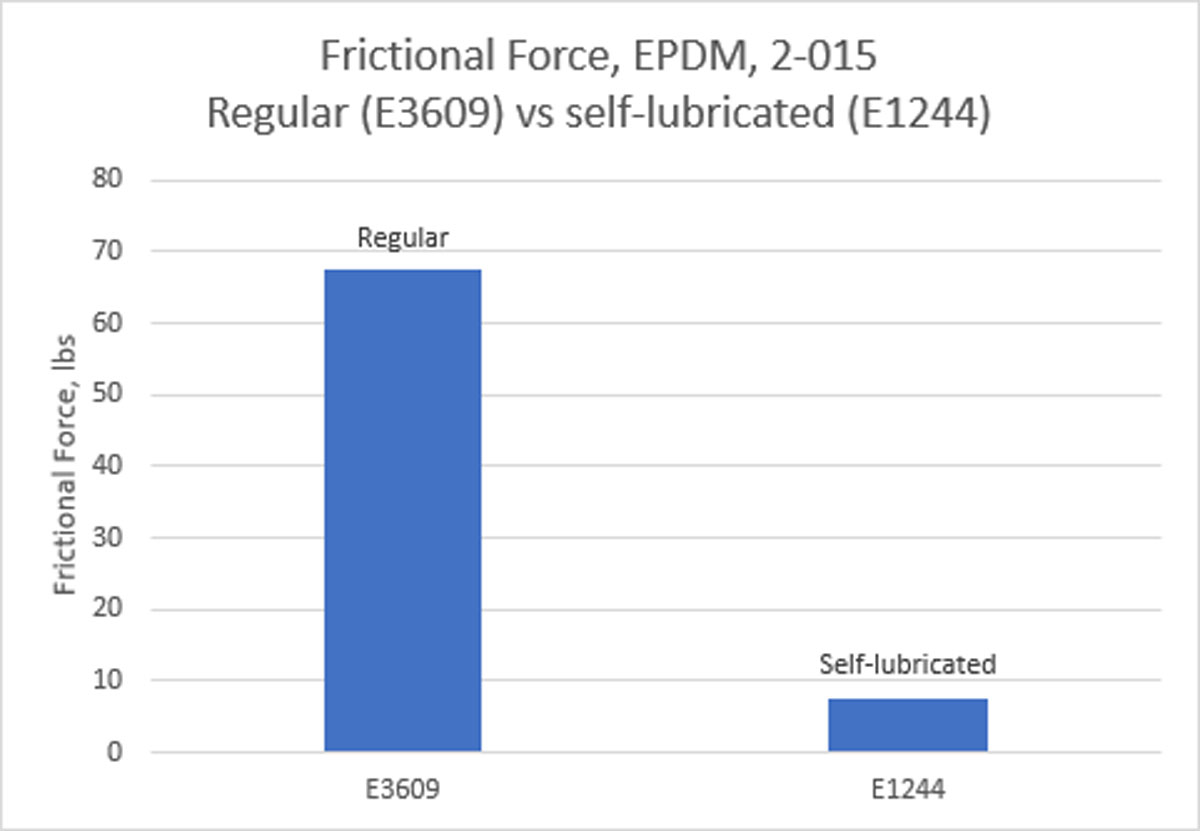
Technical Solution Details:
- Internally Lubed Compounds: The innovative use of internally lubricated EPDM compounds provided a solution that integrated seamlessly with the existing device design, eliminating the need for design modifications.
- Regulatory Compliance: The selected EPDM line, including the initially trialed E1244-70 and the later adopted EJ243-45, received FDA and USP Class VI approvals, making them suitable for a wide range of Life Science applications.
- Adjustment and Optimization: Despite the initial selection of the E1244-70 compound, its hardness posed challenges during installation. The pivot to the softer EJ243-45 compound resulted in an optimized balance between ease of installation and performance, leading to consistent and reliable drug delivery.
The implementation of Parker OES's internally lubricated sealing solutions effectively resolved the friction-related challenges faced by the wearable drug delivery device manufacturer. By maintaining low friction levels throughout the device's operational life without compromising Life Science compliance, the manufacturer achieved consistent drug delivery, enhancing device reliability and patient outcomes.
This case exemplifies the critical role of innovative sealing solutions in addressing complex challenges within the Life Science sector. Parker OES's internally lubricated compounds offer a viable pathway to overcome design limitations and friction management issues, ensuring that wearable drug delivery devices meet both performance and regulatory requirements. To learn more, register for our drug delivery tech webinar below to discuss your project needs with a Parker engineer, please visit Parker OES and chat with us.
Tech Webinar: Optimizing Drug Delivery Devices for Longevity and Sustainability with Advanced Sealing Solutions
Listen to Parker's industry leaders in this exclusive webinar, meticulously designed for professionals in drug delivery manufacturing. Discover how our certified, precision-engineered sealing solutions, including state-of-the-art O-rings, enclosure seals, and custom molded seals, are setting new benchmarks in the Life Sciences sector. This is a pivotal opportunity for drug delivery device manufacturers to explore Parker OES's extensive capabilities and how they can dramatically enhance device reliability and performance. Elevate your project's success with insights from the forefront of sealing technology. Secure your spot today and lead the way in innovative drug delivery solutions.
© 2023 Parker Hannifin Corporation
O-Ring & Engineered Seals Division
2360 Palumbo Drive
Lexington, KY 40509